Government Customers
Contract & Organization Information
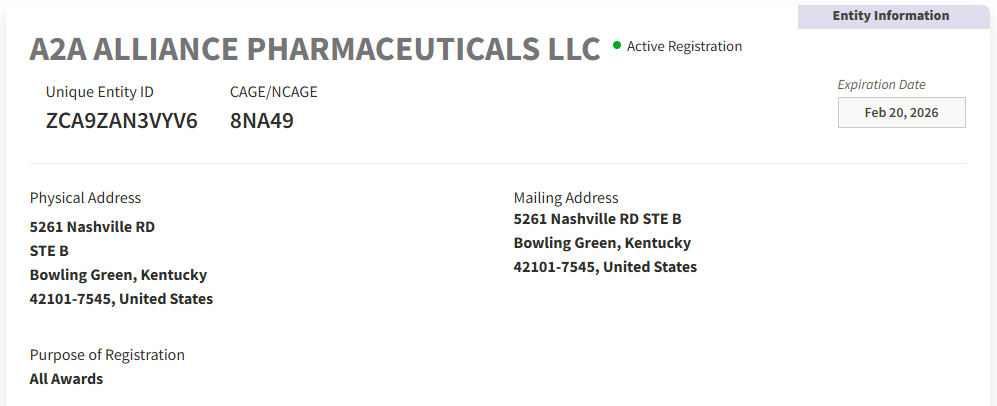
A2A Alliance Pharmaceuticals, LLC
DUNS (Official Unique Entity ID): 117589989
CAGE Code: 8NA49
SAM Unique Entity ID: ZCA9ZAN3VYV6
SAM Expiration Date: Feb 20, 2026
CVE-SDVOSB Expiration Date: Dec 16, 2026
Federal Supply Schedule (FSS)
FSS (65IB) Pharma: 36F79722D0086
Active Date: 3/15/2022-3/14/2027

Defense Logistics Agency (DAPA)
DAPA#: SP020021H0058
Active Date: 7/31/2021-12/30/2030
Government Customers

Department of Veterans Health Affairs (VA/VHA)
- Veterans Integrated Services Network (VISN)
- VA National Acquisition Center (NAC)

Department of Defense (DOD)
- Defense Logistics Agency (DLA)
- Tricare (DHA)
- US Army National Guard (ARNG)
Indian Health Services (IHS)
Health & Human Services (HHS)
Food & Drug Administration (FDA)
State & Local Entities
Wholesalers & (Prime Vendor) Distribution Partners




A2A Customer Network
The U.S. Federal Channels primarily refers to the Department of Veterans Affairs (VA) healthcare program managed by the Veterans Health Administration (VHA) and the Tricare (DOD) healthcare program managed by the Defense Health Agency (DHA). In addition to the larger U.S. Government Agencies, our customer network encompasses many other agencies, programs, and contracts at all levels of the acquisition and procurement process through Prime Vendor programs as well as indirect methods to supply products and services to end-users in the U.S. Government.

A2A Supplier Network
A2A Alliance Pharmaceuticals, LLC (a Certified Service-Disabled Veteran Owned Organization), a Pharmaceutical Wholesale/Distribution and Manufacturing organization has developed the strategic "A2A Network" with 100+ years of Pharma Industry at top levels and U.S. Federal experience with high level security clearances with the mission to provide innovative and FDA-approved products to our US Military colleagues and Veterans, such as members of our organization. Our capabilities include many aspects of the pharmaceutical supply chain from manufacturing, regulatory & quality, marketing services to strategic distribution and contract management and software services. A2A utilizes an eQMS that is considered the #1 “e” Quality Management System to bring traditional regulatory and quality aspects required by the FDA for pharmaceutical manufacturing organizations into the “new age”. This allows for the A2A Regulatory & Quality team to provide FDA and cGMP required compliance to be scaled without sacrificing results or product risks. A2A has strict supplier vetting requirements that include Supplier Audits, Quality Agreements, and other compliance related agreements to allow compliance with FDA and cGMP guidelines, guidances, and requirements. Each product has a strict regulatory and quality approval process to maximize quality and regulatory compliance to maintain the standards set forth in A2A’s Quality Policy.

Proprietary Digital Ecosystem
The proprietary digital ecosystem that encompasses the A2A Network provides a transparent, end to end pharmaceutical manufacturing and supply chain knowledge base. The quality and regulatory aspects of the digital ecosystem is a pillar to ensure the safety and quality control concepts of the pharmaceutical supply chain.
Contact a Sales Rep or Setup an Account!
